

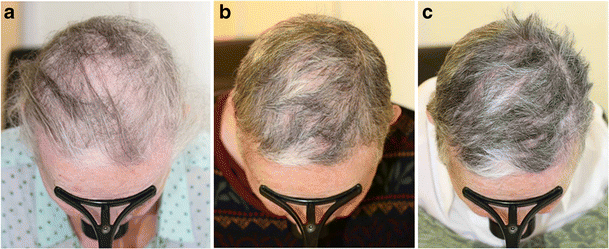
And the lifetime incidence risk of AA is approximately 2% ( Mirzoyev et al., 2014). As a common type of alopecia in human, the estimated prevalence of AA is approximately between 0.1% and 0.2%, second only to male and female pattern alopecia ( Pratt et al., 2017). The risk of progression from patchy AA to AT or AU is approximately 5%, and an extensive involvement portends a worse prognosis ( Safavi et al., 1995 Tosti et al., 2006). AA presents most commonly as limited patches of hair loss (patchy AA) that can progress to loss of all scalp hairs (alopecia totalis, AT) or all body hairs (alopecia universalis, AU) ( Strazzulla et al., 2018). Systematic Review Registration: PROSPERO: CRD 42022303007Īlopecia areata (AA) is a common, inflammatory, non-scarring type of hair loss. Due to the high recurrence rate after withdrawal of JAK inhibitors, continuous treatment should be considered to maintain efficacy. AT/AU) are associated with better efficacy outcomes in non-RCT. Subgroup analyses indicate that baricitinib, ritlecitinib and brepocitinib seem to have equal efficacy for AA in RCTs ruxolitinib (vs. In RCTs, no difference was found in the risk of experiencing most kind of adverse events in non-RCTs, the reported adverse events with high incidence rate were mostly mild and manageable.Ĭonclusion: JAK inhibitors are efficacious and generally well-tolerated in treating AA with oral administration, whereas topical or sublingual administration lacks efficacy. The pooled recurrence rate in patients treated with JAK inhibitors was 54% (95% CI: 39%–69%), mainly due to the withdrawal of JAK inhibitors. In non-RCTs, the pooled rate of good response to oral, topical and sublingual JAK inhibitors were 63% (95% CI: 44%–80%), 28% (95% CI: 1%–72%) and 11% (95% CI: 1%–29%), respectively.

In RCTs, oral JAK inhibitors resulted in higher good response rate compared with control (RR: 6.86, 95% CI: 2.91–16.16) topical JAK inhibitors did not show any difference compared with control (RR: 1.00, 95% CI: 0.31–3.18). Results: Fourteen prospective studies (5 RCTs and 9 non-RCTs), enrolling a total of 1845 patients with AA, were included for quantitative analysis. Methods: The systematic review and meta-analysis was performed pursuant to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guideline and registered on PROSPERO (CRD42022303007). 5Peking University People’s Hospital, Peking University Hepatology Institute, Beijing Key Laboratory of Hepatitis C and Immunotherapy for Liver Disease, Beijing, Chinaīackground: Due to the lack of comprehensive evidence based on prospective studies, the efficacy and safety of Janus Kinase (JAK) inhibitors (including tofacitinib, ruxolitinib, baricitinib, ritlecitinib and brepocitinib) for alopecia areata (AA) are yet to be proved.
4Department of Clinical Pharmacy, Xuzhou Medical University, Xuzhou, China.3Department of Science and Research, Peking University People’s Hospital, Beijing, China.


 0 kommentar(er)
0 kommentar(er)
